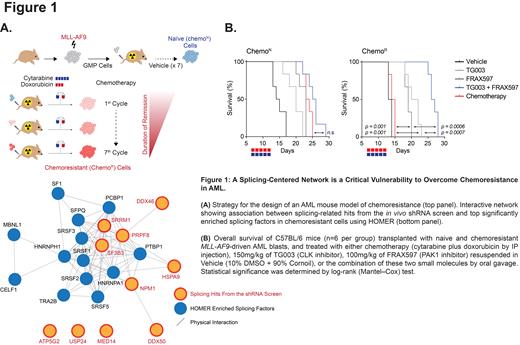
In Acute Myeloid Leukemia (AML), distinguishing between chemoresistance driver mechanisms and cell plasticity in response to chemotherapy is challenging due to their interconnectedness. To address this issue, we generated an MLL-AF9-driven syngeneic mouse model of AML, which we evolved to develop resistance to a front-line chemotherapy regimen by repetitive exposure to a maximally-tolerated combination of cytarabine and doxorubicin. An RNA sequencing (RNAseq)-based profiling of the chemoresistant (Chemo R) AML subpopulation and its naive (Chemo N) counterpart revealed a significant upregulation in the expression of 326 genes in Chemo R cells which were markedly enriched in gene sets related to splicing/mRNA processing. Notably, single-sample GSEA analysis of relapsed AML samples from the BEAT-AML cohort supported these findings, as they were enriched for the upregulation of genes in our chemoresistance gene signature. A combined in vivo functional screening endeavor using a pool of 2098 shRNAs targeting these upregulated genes and a motif discovery analysis performed on the set of dysregulated transcripts in Chemo R cells identified three candidate hits, Srrm1, Sf3b3, and Prpf8, which scored as chemotherapy-specific dependencies and were found to interact with central regulators of alternative splicing ( Figure 1A). Silencing these three candidate genes in our chemoresistant mouse model and the human AML cell lines, MOLM-14, MV4-11, and U937, evolved to become resistant to the combination of cytarabine and daunorubicin, revealed that the splicing regulator, SRRM1, is a critical vulnerability of chemoresistant leukemic cells.
An orthogonal phospho-proteomic approach identified that PAK1 and CLK kinases are regulators of SRRM1 in chemoresistant AML cells, through the phosphorylation of multiple serine and threonine phosphosites in its arginine-rich domain. Human and murine chemoresistant cells were found to be markedly more sensitive to inhibitors of PAKs (FRAX597) and CLKs (TG003 and ML167) compared to their naive counterparts, an effect which was even further enhanced by treatment with cytarabine and daunorubicin. Notably, a whole-exome sequencing pipeline at our institution revealed a clonal variant of uncertain significance (c.1429G>T p.(Ala477Ser)) in PAK1 in an AML patient at relapse. Absence of this variant at diagnosis suggested its association with chemotherapy resistance. Biochemical and cellular assays confirmed that the A477S variant increased PAK1 activation as well as chemotherapy resistance in AML cell lines. This effect could be counteracted by suppressing SRRM1, indicating that PAK1 activation in relapsed AML confers increased vulnerability to SRRM1 suppression.
Simultaneous suppression of Clk1 and Pak1 or Clk4 and Pak1 using validated shRNAs and combinations of FRAX597 and TG003, or FRAX597 and ML167 synergized to reduce the growth and the colony-forming capacity of chemoresistant AML cells and sensitized them to chemotherapy. These observations were supported by the RNAseq-based estimate of the inclusion ratios of alternative exons and introns which showed that the combination of PAK and CLK inhibitors phenocopied partially the effects of SRRM1 silencing on splicing perturbations. In preclinical studies, the combined treatment improved overall mouse survival and reduced disease burden in our chemoresistant AML mouse models compared to single-drug treatments ( Figure 1B). Moreover, AML patient cells that were primarily refractory or from a post chemotherapy relapse (n=21) exhibited a higher ex vivo sensitivity to the FRAX597 + TG003 combination in comparison with chemosensitive patients at diagnosis (n=28). Moreover, in NSG-S mice transplanted with matched samples from the same AML patient at diagnosis and relapse post-chemotherapy, combined PAK1 and CLK inhibition more effectively reduced disease progression in animals transplanted with the relapse sample than in those engrafted with the sample taken at diagnosis.
In conclusion, our study advances the rationale that combined inhibition of PAK1 and CLK kinases effectively curtails malignant cell splicing adaptation to chemotherapy by affecting SRRM1 function. Combined PAK1 and CLK inhibitors either concomitant with or sequential to chemotherapy may offer greater clinical benefit and significantly improve AML patients' long-term outcomes.
Disclosures
Raffoux:Celgene: Honoraria; Pfizer, Inc.: Honoraria; Daiichi-Sankyo: Honoraria; AbbVie: Honoraria; Astellas: Honoraria. Benajiba:Gilead: Research Funding; Pfizer: Research Funding. Stegmaier:Auron Therapeutics: Current holder of stock options in a privately-held company; Novartis: Research Funding; Kronos Bio: Research Funding. Wood:Guidepoint Global: Consultancy; Tavros Therapeutics: Consultancy, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Celldom: Consultancy, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Simple Therapeutics: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Decrypt Biomedicine: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Bantam Pharmaceuticals: Consultancy; Apple Tree Partners: Consultancy. Puissant:Amgen: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal